Factors Affecting the Boiling Time of Water
There are many things which affect the boiling of water. So in this post we will learn like How Long Does Water Take to Boil. So let’s get started

When it comes to boiling water, several factors can affect the amount of time it takes for the water to reach its boiling point. Understanding these factors will help you better control and predict the boiling time for your water.
One of the key factors is the altitude and atmospheric pressure. At higher altitudes, the boiling point of water decreases due to lower atmospheric pressure. This means that it will take longer for water to reach its boiling point at higher elevations compared to sea level.
Another factor is the quantity of water and the size of the container. Larger quantities of water will take longer to heat up and boil compared to smaller amounts. Similarly, using a larger container can also increase the boiling time since more heat will be needed to raise the temperature of the water.
The starting temperature of the water also plays a role. If the water is already warm or hot, it will take less time to heat up and reach its boiling point. On the other hand, if the water is starting from room temperature, it will take longer to boil.
Additionally, the heat source used can also affect the boiling time. Different heat sources such as a stove, microwave, or kettle can have varying heating capacities, which in turn will affect the time it takes for the water to boil.
By considering these factors, you can adjust your boiling time accordingly. Whether you’re cooking pasta or making a hot beverage, understanding the factors affecting boiling time will help you plan and manage your cooking process more efficiently.
A. Altitude and atmospheric pressure
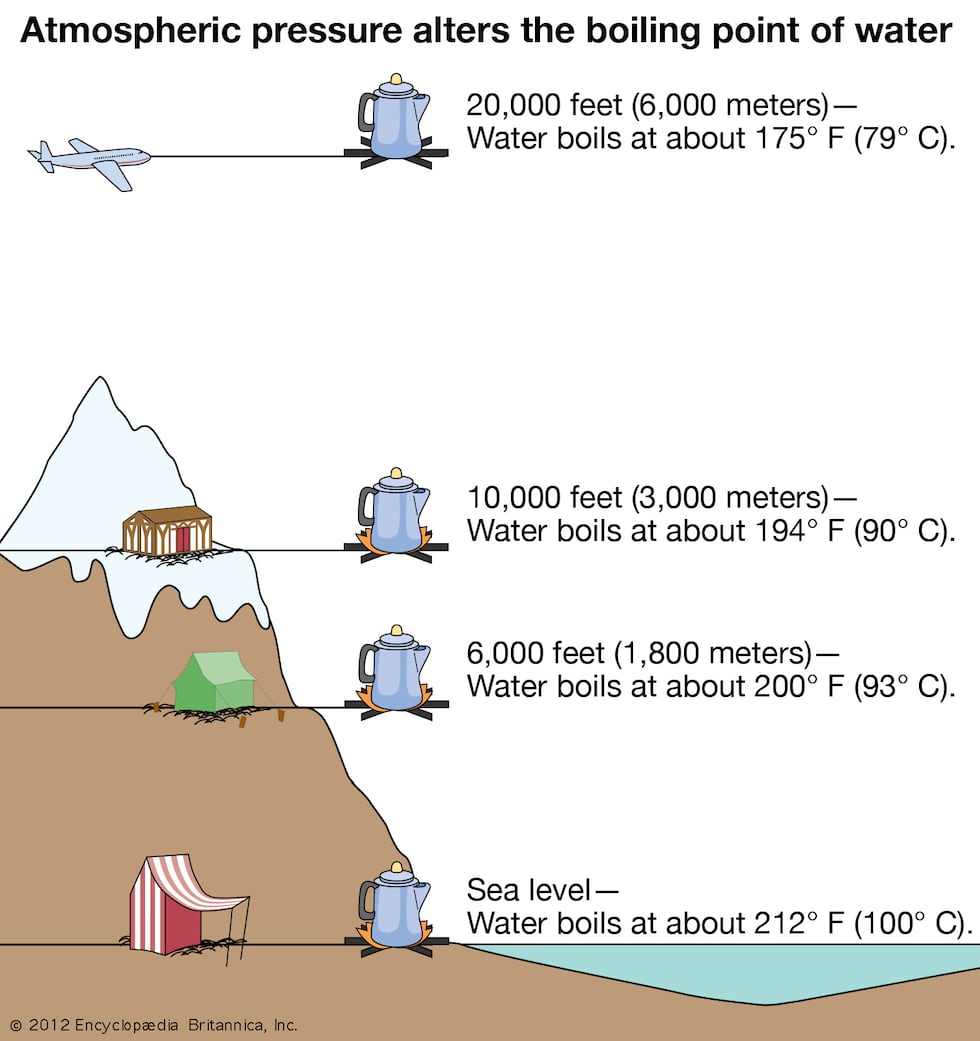
At higher altitudes, the atmospheric pressure decreases, which has a direct effect on the boiling point of water. The relationship between altitude and boiling point is due to the fact that boiling occurs when the vapor pressure of the liquid equals the pressure of the surrounding atmosphere.
As you go higher in altitude, the atmospheric pressure decreases, meaning that the pressure on the surface of the water is also reduced. This reduced pressure lowers the boiling point of water, as less heat energy is required to reach the vapor pressure needed for boiling.
At sea level, where the atmospheric pressure is the highest, water boils at 100 degrees Celsius (212 degrees Fahrenheit). However, at higher altitudes, such as 5000 feet above sea level, the boiling point of water decreases to approximately 95 degrees Celsius (203 degrees Fahrenheit).
This means that water will boil at a lower temperature and take less time to reach its boiling point at higher altitudes. The lower boiling point can have implications for cooking and baking, as recipes may need to be adjusted to account for the lower boiling point of water.
It’s important to understand the relationship between altitude and boiling point, especially if you plan to cook or bake at high elevations. By considering altitude and atmospheric pressure, you can accurately determine the boiling time for water and make appropriate adjustments in your cooking process.
B. Quantity of water and size of the container

The quantity of water and the size of the container can also impact the boiling time. Here’s how these factors come into play:
- Quantity of water: The more water you have, the longer it will take to reach the boiling point. This is because a larger volume of water requires more heat energy to raise its temperature. So, if you’re boiling a small amount of water, such as one or two cups, it will reach the boiling point faster compared to a larger quantity like several quarts or liters.
- Size of the container: The size of the container affects the heating efficiency. A smaller container will heat up faster due to its smaller surface area and closer proximity to the heat source. On the other hand, a larger container will heat up more slowly as it has a larger surface area for heat to dissipate.
To save time while boiling water, consider these tips:
- Use the right-sized pot or kettle for the amount of water you need. Using a small pot for a small quantity will speed up the boiling process.
- If you need to boil a large quantity of water, consider using a lid to trap the heat and reduce heat loss through evaporation.
- Opt for a container with a wide base and tall sides, as this shape allows for better heat transfer and quicker boiling.
- Consider using a kettle with a higher wattage if you frequently need to boil water quickly.
Remember, the quantity of water and the size of the container are important factors to consider when estimating boiling time. By understanding their impact, you can plan your cooking or beverage preparation more efficiently.
Boiling Time of Water at Sea Level
:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__serious_eats__seriouseats.com__images__20100813-boling-water-timeline-aa32b07cdfe1451aba973a53d5c89ab2.jpg)
At sea level, the boiling time of water is typically 5-10 minutes when using a stove on high heat. This is because at sea level, the atmospheric pressure is approximately 1 atmosphere (atm), which means that water boils at 100 degrees Celsius (212 degrees Fahrenheit).
When you apply heat to the water, the temperature starts to rise, and once it reaches the boiling point, bubbles begin to form and it starts to boil. The time it takes to reach this point depends on the starting temperature of the water, the amount of water in the pot, and the efficiency of the stove or heat source.
Using a pot or kettle with a wider base and taller sides can help to facilitate better heat transfer and more efficient boiling. Additionally, using a lid on the pot can trap the heat and reduce heat loss through evaporation, which can speed up the boiling process.
If you need to boil water for specific purposes, such as cooking pasta, it is important to follow the cooking instructions provided. Most recipes will specify the boiling time necessary for achieving the desired result.
Moreover, it’s worth noting that the boiling time can vary slightly depending on the type of stove or heat source being used. Gas stoves tend to heat up faster than electric stoves, so you may notice a difference in boiling times between the two. Therefore, it is important to adjust the cooking time accordingly.
Overall, at sea level, the boiling time of water ranges from 5-10 minutes, but factors such as the size of the container, the starting temperature of the water, and the efficiency of the heat source can impact the boiling time. By understanding these factors, you can better plan your cooking and beverage preparation.
A. Standard boiling time of water at sea level
:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__serious_eats__seriouseats.com__images__20100813-boling-water-timeline-aa32b07cdfe1451aba973a53d5c89ab2.jpg)
At sea level, the standard boiling time for water is typically 5-10 minutes. This is based on using a stove on high heat. The boiling point of water at sea level is 100 degrees Celsius (212 degrees Fahrenheit), which is the temperature at which water turns into vapor and starts to boil.
When you apply heat to the water, the temperature begins to rise until it reaches the boiling point. Once it reaches this point, bubbles start to form and the water starts to boil. The time it takes for the water to reach the boiling point depends on factors such as the initial temperature of the water, the amount of water in the pot, and the efficiency of the stove or heat source.
To achieve the standard boiling time of 5-10 minutes, it is important to use a pot or kettle with a wider base and taller sides. This design facilitates better heat transfer and more efficient boiling. Additionally, using a lid on the pot can trap the heat and reduce heat loss through evaporation, which can speed up the boiling process.
It is worth noting that the boiling time can slightly vary depending on the type of stove or heat source being used. Gas stoves tend to heat up faster than electric stoves, so there may be a difference in boiling times between the two. It is important to adjust the cooking time accordingly.
In summary, at sea level, the standard boiling time for water ranges from 5-10 minutes. Factors such as the size of the container, the starting temperature of the water, and the efficiency of the heat source can impact the boiling time. By understanding these factors, you can plan your cooking and beverage preparation accordingly.
B. Effects of additional factors on boiling time at sea level

When it comes to boiling water at sea level, there are several additional factors that can affect the boiling time. These factors include:
- Initial temperature of the water: The colder the water, the longer it will take to reach the boiling point. Starting with hot or warm water can significantly reduce the boiling time.
- Heat source efficiency: The efficiency of the heat source used, whether it’s a gas stove or an electric stove, can impact the boiling time. Gas stoves generally heat up faster than electric stoves, so using a gas stove can help reduce the boiling time.
- Size and shape of the pot: The size and shape of the pot also play a role in the boiling time. A wider and shallower pot will have a larger surface area, allowing for faster heat transfer and quicker boiling.
- Altitude: While altitude affects the boiling time more significantly at higher elevations, it can still have a slight impact at sea level. The lower atmospheric pressure at higher elevations allows water to boil at a lower temperature, but at sea level, this impact is minimal.
- Lid on or off: Using a lid on the pot can help trap the heat and reduce heat loss through evaporation, which can speed up the boiling process. Keeping the lid on can decrease the boiling time compared to leaving it off.
- Water impurities: If the water contains impurities such as salt or other minerals, it can slightly raise the boiling point and increase the boiling time.
By considering these additional factors and making adjustments accordingly, you can achieve faster boiling times when heating water at sea level. Remember to use hot or warm water, choose an efficient heat source, use a pot with a wide base, and consider using a lid to reduce heat loss.
Boiling Time of Water at Higher Altitudes

Boiling water at higher altitudes can be a bit more challenging compared to sea level due to the lower atmospheric pressure. The reduced pressure affects the boiling point of water, causing it to boil at a lower temperature. As a result, it takes longer for water to come to a boil at higher altitudes.
For instance, at an altitude of 5000 feet above sea level, water boils at around 202°F (94°C) and may take approximately 15-20 minutes to reach the boiling point. This is because the lower air pressure hinders the transfer of heat energy into the water, requiring more time for the water to absorb enough heat to boil.
When cooking or preparing meals at higher altitudes, it’s important to adjust your cooking time and methods accordingly. Here are some tips for boiling water at higher altitudes effectively:
- Start with hot or warm water: Since it takes longer to bring cold water to a boil at higher altitudes, starting with hot or warm water can significantly reduce the boiling time.
- Increase the heat source: Using a higher heat setting on your stove or increasing the power level on your electric kettle can help compensate for the lower boiling temperature and speed up the boiling process.
- Use a lid: Covering the pot with a lid can help trap the heat and reduce heat loss through evaporation, allowing the water to reach its boiling point more quickly.
- Adjust cooking times: When following recipes or cooking instructions, it’s important to adjust the cooking times to accommodate the lower boiling temperature at higher altitudes. Consider adding a few extra minutes to the cooking time for optimal results.
By making these adjustments, you can ensure that your water reaches its boiling point efficiently, even at higher altitudes. Remember to stay vigilant and monitor the boiling process closely to prevent any potential boil-overs.
A. Impact of altitude on the boiling point of water
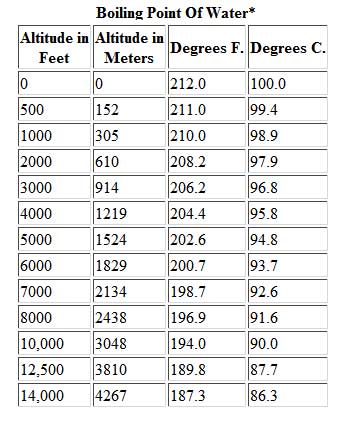
When it comes to boiling water, altitude plays a significant role in determining the boiling point. At higher altitudes, such as in mountainous regions, the boiling point of water decreases due to the decrease in atmospheric pressure. This means that water boils at a lower temperature compared to sea level.
The reason behind this phenomenon lies in the pressure exerted on the water’s surface. At sea level, the atmospheric pressure is higher, which increases the boiling point of water to 212°F (100°C). However, as you climb to higher altitudes, the atmospheric pressure decreases, causing the boiling point to drop as well.
For every 500 feet increase in altitude, the boiling point of water decreases by approximately 1°F (0.6°C). This means that at an altitude of 5,000 feet, the boiling point of water is reduced to around 202°F (94°C).
The lower boiling point at higher altitudes can have implications for cooking and food preparation. It may take longer for water to reach its boiling point, resulting in increased cooking times. Additionally, the lower boiling temperature can affect the texture and taste of certain foods.
Therefore, when cooking or preparing meals at higher altitudes, it is important to make adjustments to cooking times and temperature settings. By understanding the impact of altitude on the boiling point of water, you can ensure optimal cooking results and make necessary adaptations to your recipes.
B. Adjusting boiling time for higher altitudes

When cooking or preparing meals at higher altitudes, it is important to make adjustments to boiling time in order to ensure that your food is cooked properly. Due to the lower boiling point of water at higher altitudes, the standard boiling time used at sea level may not be sufficient. Here are some tips for adjusting boiling time at higher altitudes:
- Increase boiling time: Since the lower atmospheric pressure at higher altitudes results in a lower boiling point, you will need to increase the boiling time to compensate. As a general rule, for every 500 feet increase in altitude, you should add an additional 1 minute to the boiling time. For example, if a recipe calls for boiling pasta for 10 minutes at sea level, you may need to boil it for 15 minutes at an altitude of 5,000 feet.
- Monitor the texture: It is important to monitor the texture of your food while it is boiling. The lower boiling temperature at higher altitudes may affect the texture of certain foods, such as vegetables and grains. Keep a close eye on the firmness or tenderness of your food and adjust the boiling time accordingly.
- Use a thermometer: To ensure that your food is cooked to the desired temperature, consider using a food thermometer. This will help you determine the doneness of meats and other cooked foods, especially when the boiling time needs to be adjusted.
- Experiment and observe: Cooking at higher altitudes may require some experimentation and observation to find the optimal boiling time for your specific location. Keep a record of your cooking times and adjust as needed based on your own observations and preferences.
By making these adjustments to boiling time, you can ensure that your food is cooked thoroughly and to the right consistency, even at higher altitudes. Enjoy your delicious meals with confidence, knowing that you have adapted your cooking methods to the unique conditions of your environment.
Boiling Time of Different Quantities of Water
:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__serious_eats__seriouseats.com__images__20100813-water-boiling-leidenfrost-aa78bd323a3c41f2893c1fb7b8d94dd4.jpg)
When it comes to boiling different quantities of water, the time it takes can vary. The size of the container and the amount of water in it play a significant role in determining the boiling time. Here’s what you need to know:
A. Boiling time for small quantities of water: If you’re boiling just a few cups or a small amount of water, it will generally heat up and reach boiling point much faster than larger quantities. In a small pot or kettle, it could take as little as 2-3 minutes for the water to start boiling.
B. Boiling time for large quantities of water: When you’re dealing with larger quantities, such as several quarts or liters of water, the boiling time will naturally be longer. It can take anywhere from 10-15 minutes or even longer for the water to come to a rolling boil.
It’s important to note that the heat source and the power of the stove or burner can also affect the boiling time. Higher heat settings will typically expedite the boiling process.
To save time when boiling larger quantities of water, you can consider using a kettle, which is designed to heat up water quickly. It’s also a good idea to cover the pot or container with a lid to retain the heat and speed up the boiling process.
Remember to always monitor the water closely and keep an eye out for signs of boiling, such as bubbles forming and rising to the surface. Adjust the heat as needed to ensure a steady boil.
In summary, smaller quantities of water will generally reach boiling point faster, while larger quantities will take more time. Use the appropriate size container for the amount of water you’re boiling and adjust the heat accordingly for efficient boiling.
A. Boiling time for small quantities of water
:max_bytes(150000):strip_icc()/pot-883036_1920-5843562a5f9b5851e5745d27.jpg)
Boiling smaller quantities of water can be a quicker process compared to larger amounts. When dealing with just a few cups or a small amount of water, it will generally heat up and reach boiling point much faster. In fact, in a small pot or kettle, it could take as little as 2-3 minutes for the water to start boiling.
The reason behind this is that smaller quantities of water have less mass and require less energy to heat up. The heat from the stove or burner is concentrated in a smaller area, allowing the water molecules to gain energy more efficiently. This results in a faster rise in temperature and the water reaching its boiling point.
To further speed up the boiling time for small quantities of water, using a smaller pot or kettle can help. The smaller surface area allows for quicker heat transfer, reducing the time it takes for the water to reach a rolling boil.
Additionally, it’s important to note that the power of the stove or burner can also affect the boiling time. Higher heat settings will typically expedite the boiling process, so be mindful of adjusting the heat accordingly.
Overall, boiling small quantities of water is a relatively quick process, taking only a few minutes to bring the water to a rolling boil. This makes it ideal for tasks such as making a single cup of tea or coffee in a hurry.
B. Boiling time for large quantities of water

When dealing with large quantities of water, such as several quarts or liters, the boiling time can increase significantly compared to smaller amounts. The reason behind this is that larger quantities of water have more mass and require more energy to heat up. This means that it will take longer for the water to reach its boiling point.
On a stove set to high heat, it can take around 10-15 minutes or even longer for a large pot of water to come to a rolling boil. The exact time will depend on factors such as the heat source, the starting temperature of the water, and the size and shape of the container.
To expedite the boiling time for large quantities of water, there are a few tips you can follow. First, make sure to use a large pot or kettle that can accommodate the amount of water you need. A pot with a wide bottom and a good amount of surface area can promote faster heat transfer and reduce the boiling time.
Additionally, covering the pot with a lid can help trap the heat and increase the efficiency of the boiling process. By keeping the heat contained, the water can reach its boiling point faster.
It’s important to note that with larger quantities of water, it’s also crucial to be mindful of safety precautions, such as avoiding overfilling the pot to prevent spills and burns.
Overall, boiling large quantities of water will take more time compared to smaller amounts. By using a large pot, covering it with a lid, and using a high heat source, you can reduce the boiling time and efficiently heat up a larger volume of water.
Boiling Time in Different Containers
:max_bytes(150000):strip_icc()/GettyImages-155146092-2000-11ed7496811e4ea4b6d08ab6aee1f3b9.jpg)
When it comes to boiling water, the type of container you use can also have an impact on the boiling time. Different containers have varying levels of heat conductivity and can affect how efficiently the water heats up. Here’s what you need to know about boiling time in different containers:
A. Boiling time in a pot with a lid:
- Using a pot with a lid can help trap the heat and reduce the boiling time. The lid helps to contain the heat within the pot, allowing the water to reach its boiling point faster.
- With a lid, it typically takes around 5-7 minutes for a pot of water to come to a rolling boil on a stove over high heat, similar to the boiling time at sea level. The lid helps to retain the heat, speeding up the process.
- Using a pot with a wide bottom and a good amount of surface area can promote faster heat transfer, further reducing the boiling time.
B. Boiling time in an open pan:
- If you’re boiling water in an open pan or a pot without a lid, the boiling time can be slightly longer compared to using a pot with a lid.
- Without the lid, some of the heat escapes, resulting in a slightly longer boiling time. It can take around 7-10 minutes for the water to reach its boiling point in an open pan.
- To speed up the boiling process in an open pan, you can increase the heat slightly or use a pan with a wider bottom to increase the surface area for heat transfer.
In summary, using a pot with a lid can help reduce the boiling time by trapping the heat and promoting faster heat transfer. However, even without a lid, boiling water in an open pan is still a viable option, albeit with a slightly longer boiling time. Choose the right container based on your preferences and the convenience of your cooking setup.
A. Boiling time in a pot with a lid
:max_bytes(150000):strip_icc()/GettyImages-155146092-2000-11ed7496811e4ea4b6d08ab6aee1f3b9.jpg)
When it comes to boiling water, using a pot with a lid can significantly impact the boiling time. This is because a lid helps to trap the heat inside the pot, allowing the water to come to a boil faster. If you’re looking to expedite the boiling process, using a pot with a lid is highly recommended.
The lid on a pot creates a sealed environment, preventing heat from escaping and increasing the heat transfer efficiency. As a result, the water reaches its boiling point much quicker compared to an open pan or pot without a lid.
On a stove set to high heat, it typically takes around 5-7 minutes for a pot of water to come to a rolling boil when using a lid. This boiling time is similar to what you would expect at sea level.
In addition to using a lidded pot, consider using one with a wide bottom and a good amount of surface area. This promotes faster heat transfer, further reducing the boiling time. A pot with a wide bottom allows for more direct contact between the heat source and the water, facilitating efficient heat transfer.
By using a pot with a lid and a wide bottom, you can significantly shorten the time it takes to bring water to a boil. This can be particularly useful when you’re in a hurry or need boiling water for time-sensitive recipes. Don’t forget to securely place the lid on the pot to maximize heat retention.
In conclusion, when it comes to boiling water, using a pot with a lid is a practical choice. It helps retain heat, promotes faster heat transfer, and ultimately reduces the boiling time. So, make sure to cover your pot for quicker boiling results.
B. Boiling time in an open pan

When boiling water in an open pan, the boiling time can be slightly longer compared to using a pot with a lid. This is because without a lid, heat is able to escape more easily, resulting in slower heat transfer and a longer boiling process. Here are some key factors to consider when boiling water in an open pan:
- Heat conductivity: The type of material used in the pan can affect how quickly heat is transferred to the water. Materials with higher heat conductivity, such as copper or aluminum, can heat up the water faster than materials with lower heat conductivity, such as stainless steel or cast iron.
- Surface area: A pan with a wider surface area allows for greater contact between the heat source and the water, promoting faster heat transfer and reducing boiling time. Opt for a wider and shallower pan instead of a narrow and deep one.
- Water quantity: The amount of water in the pan can also impact boiling time. Larger quantities of water will take longer to boil compared to smaller amounts. It is generally more efficient to boil smaller batches of water in an open pan.
- Heat source: The type of heat source being used, such as a gas burner or an electric stove, can also affect boiling time. Gas burners generally provide more direct heat, resulting in faster boiling times compared to electric stoves.
While boiling water in an open pan may take slightly longer compared to using a lidded pot, it can still be an effective method for cooking or other purposes. Just keep in mind that you may need to adjust your cooking times accordingly, and it may take a bit more patience.
Conclusion

In conclusion, the boiling time of water can be influenced by various factors, including altitude, quantity of water, and the type of container used. Understanding these factors and how they affect boiling time can help you plan your cooking and beverage preparation more effectively.
At sea level, water typically boils in about 8-10 minutes. However, this can vary depending on additional factors such as the heat source and the presence of impurities in the water. It is also important to note that boiling time increases as you go to higher altitudes due to the lower atmospheric pressure. Adjusting boiling time accordingly can help ensure that your food is cooked properly and that your beverages are heated to the desired temperature.
The quantity of water being boiled also plays a role in boiling time. Smaller quantities of water will reach boiling point faster than larger amounts. Additionally, the type of container used can impact boiling time. Using a pot with a lid can speed up the boiling process by trapping heat and preventing it from escaping.
To reduce boiling time, consider using a gas stove instead of an electric one, as gas burners generally provide more direct heat. Opt for wider and shallower pans to maximize heat transfer. Additionally, boiling smaller batches of water can be more efficient than boiling large quantities.
Overall, knowing the factors that affect boiling time and implementing practical tips can help you save time and energy in the kitchen. By understanding the science behind boiling water, you can confidently prepare your meals and enjoy a hot cup of tea or coffee in no time.
Comparison of factors affecting boiling time
When it comes to boiling water, there are several factors that can affect the overall boiling time. Let’s compare these factors and see how they influence the time it takes for water to reach its boiling point:
- Altitude and atmospheric pressure: Higher altitudes have lower atmospheric pressure, which means that water boils at a lower temperature. As a result, it takes longer for water to reach its boiling point at higher altitudes compared to sea level.
- Quantity of water and size of the container: It’s a known fact that smaller quantities of water reach boiling point faster than larger amounts. This is because there is less water to heat up. Similarly, using a wider and shallower container allows for more efficient heat transfer, resulting in faster boiling times.
- Heat source: The type of heat source used can also impact boiling time. Gas stoves tend to provide more direct heat compared to electric stoves, which can speed up the boiling process.
- Starting temperature: If the water is already warm or hot, it will reach boiling point faster than water starting at room temperature. Starting with hot water can significantly reduce boiling time.
By comparing these factors, we can see that altitude and quantity of water have the most significant impact on boiling time. However, all of these factors should be taken into consideration when determining the optimal boiling time for your needs.
Remember, these are general guidelines, and actual boiling time can vary depending on other factors such as the impurities present in the water and the efficiency of the heat source. To ensure accuracy, it’s always best to keep an eye on the water and use a thermometer if necessary.
By understanding the factors that affect boiling time and implementing practical tips, you can save time and energy in the kitchen.
Practical tips for reducing boiling time

To reduce the boiling time of water, there are several practical tips you can follow:
- Use hot water: If you need boiling water quickly, start with hot water from the tap instead of cold. This can significantly reduce the time it takes for the water to reach its boiling point.
- Cover the pot: When boiling water, always use a lid to cover the pot. This helps trap the heat and prevents it from escaping, allowing the water to heat up faster.
- Use smaller pieces of food: When cooking foods like pasta or vegetables, cut them into smaller pieces. This increases their surface area and allows them to cook faster, reducing the overall boiling time.
- Use a kettle: Using an electric kettle to boil water is often faster than using a stove. Kettles are designed to heat water more efficiently, so consider using one if you need boiling water quickly.
- Optimize your heat source: If you’re using a stovetop, make sure the burner is the appropriate size for your pot. Using a small burner for a large pot can waste energy and prolong the boiling time. Additionally, if you have a gas stove, ensure the flame is adjusted to its highest setting for faster boiling.
- Add salt to the water: Adding a pinch of salt to the water can increase its boiling point slightly, resulting in a faster boiling time. However, keep in mind that this may affect the taste of the final dish.
By implementing these practical tips, you can reduce the boiling time of water and save valuable time in the kitchen. Remember, always stay cautious when cooking with boiling water to avoid any accidents or injuries.

Frequently Asked Questions
How long does water take to boil in the microwave?
The time it takes for water to boil in a microwave can vary depending on the quantity and the power of your microwave. On average, it can take anywhere from 1-5 minutes. It is important to periodically check on the water and use caution when removing it from the microwave as it can become superheated and cause injury.
How long does water take to boil on the stove?
The time it takes for water to boil on the stove depends on various factors such as the quantity of water, the size of the pot, and the heat setting. Generally, it takes about 4-6 minutes to bring a pot of water to a rolling boil on high heat.
How long does water take to boil for pasta?
The boiling time for water used to cook pasta can vary depending on the type and thickness of the pasta. On average, it takes about 8-12 minutes, but it is advisable to follow the cooking instructions on the pasta packaging for the most accurate boiling time.
How long does water take to boil on medium heat?
Boiling water on medium heat will generally take a bit longer compared to high heat. It can take around 8-10 minutes for the water to reach a rolling boil at medium heat. Adjust the heat and cooking time accordingly based on your specific needs.
Remember to always be cautious when handling boiling water, and use the appropriate safety measures to prevent accidents or injuries. Lear more at our blog


1 thought on “Best Steps on How Long Does Water Take to Boil in 2024”